NEJM
May 20, 2015
Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults
Mazen Kherallah
Summarized by:
What was the research question?
Does permissive underfeeding with restriction of non-protein calories reduce 90-day mortality in critically ill patients compared to standard feeding?
How did they do it?
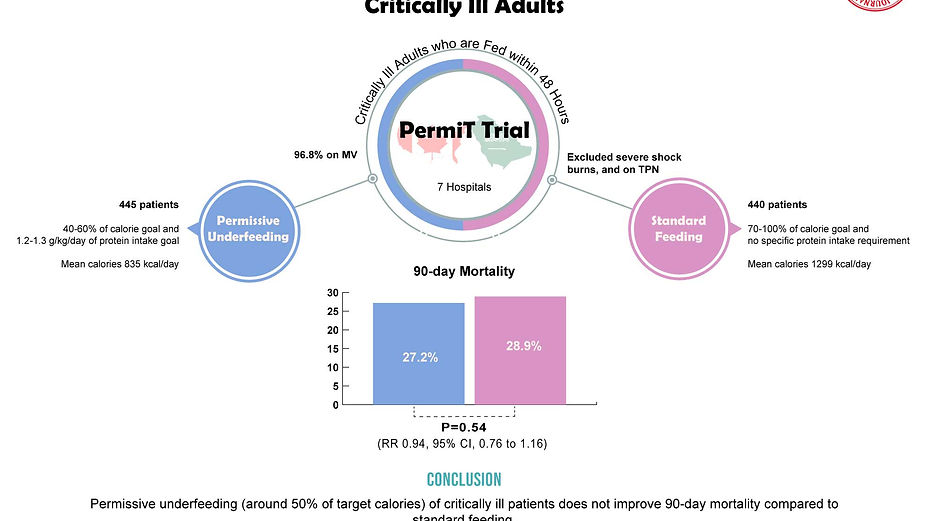
A non-blinded, randomized, controlled clinical trial in 7 hospitals the Saudi Arabia and Canada.
895 critically ill patients who began enteral feeding within 48 hours of ICU admission and expected to stay in the ICU for more than 72 hours, were randomized to receive permissive underfeeding with 40-60% of calorie goal and 1.2-1.3 g/kg/day of protein intake goal (445 patients) or standard feeding with 70-100% of calorie goal without specific protein requirement (440 patients).
Patients with high dose vasopressors (e.g. norepinephrine >0.4 mcg/kg/min), burns and those receiving TPN were excluded from the trial.
The primary outcome was 90-day mortality rate.
Secondary outcome included mortality rates at different time points (ICU mortality, hospital mortality, 28 days, and 180 days), ICU-free days, and ventilator-free days.
What did they find?
96.8% of the patients were receiving mechanical ventilation.
Patients in the permissive underfeeding group received fewer mean calories than did the standard feeding group (835 vs. 1299 kcal/day, P<0.001; 46±14% vs. 71±22% of caloric requirements, P><0.001).
90-day mortality was not significantly better in permissive underfeeding group compared to the standard group (27.2% vs. 28.9%, RR 0.94, 95% confidence interval [CI], 0.76 to 1.16; P=0.58).
Secondary endpoints were not significantly different between the two groups.
Post-hoc analysis revealed a lower renal replacement therapy rate in the permissive underfeeding group compared to the standard group (7.1% vs. 11.4%, p=0.04).
Are there any limitations?
Cannot be generalized to other patients as only 14% of screened patients were included in the study.
The non-blinded and the clinician-led management of non-nutritional care nature of the study may have introduced bias and impacted the study.
A small survival benefit cannot be ruled out as the study is powered for 8% reduction.
What does it mean?
Permissive underfeeding (around 50% of target calories) of critically ill patients does not improve 90-day mortality compared to standard feeding.
The study is not large enough to detect a smaller benefit. However, permissive underfeeding appears to be safe.